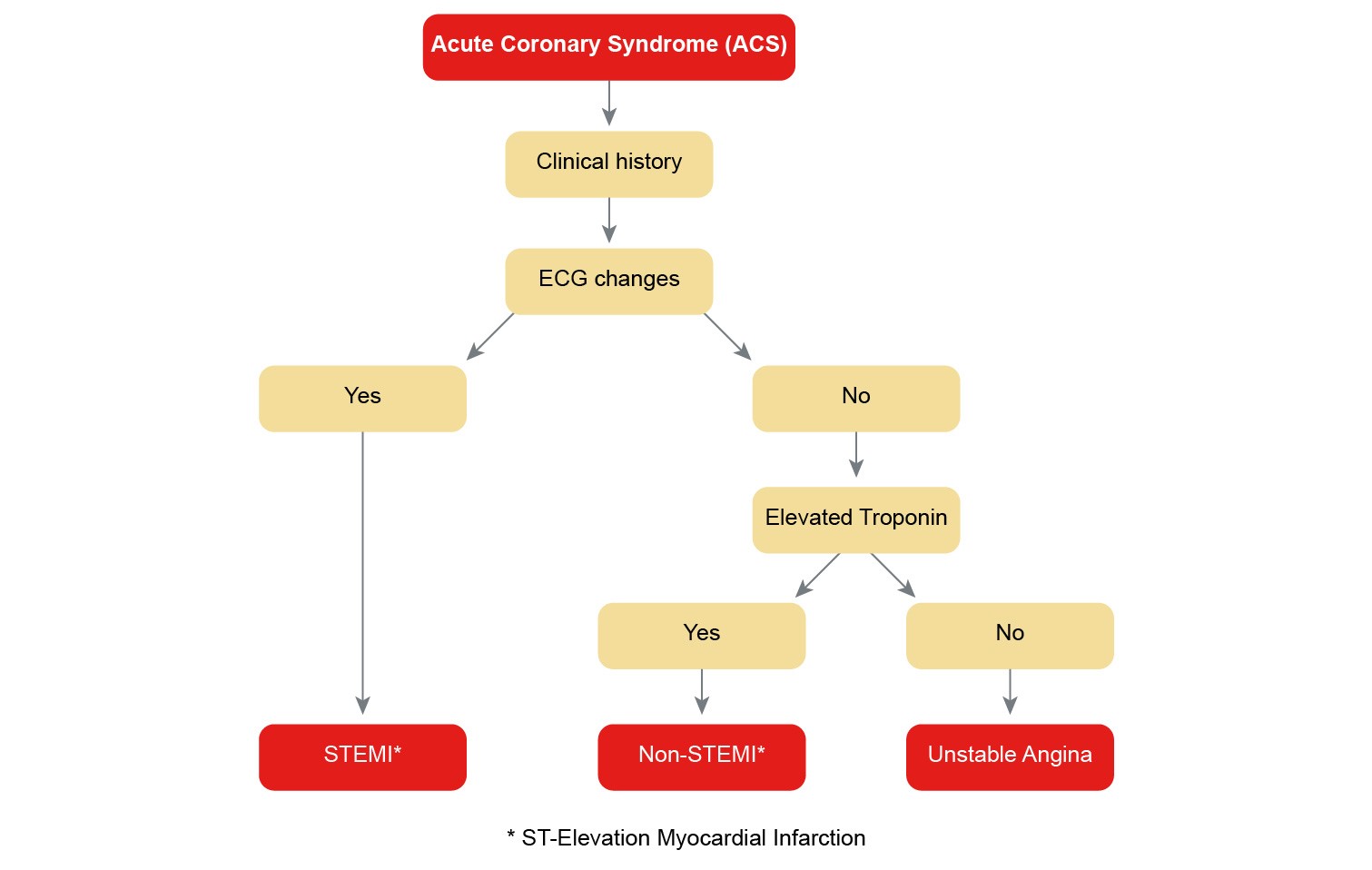
Heart failure (HF) is a debilitating condition that is characterised by shortness of breath, fatigue and exercise intolerance. HF is a clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood at rest or during physical activity. Changes to heart muscle tissue and function, neurohormonal changes, and vascular and skeletal muscle function changes are often referred to as the “vicious cycle” of heart failure as illustrated in the figure below.
Figure 2: The “vicious cycle" of heart failure pathophysiology
(Reproduced with permission from the National Heart Foundation of Australia)
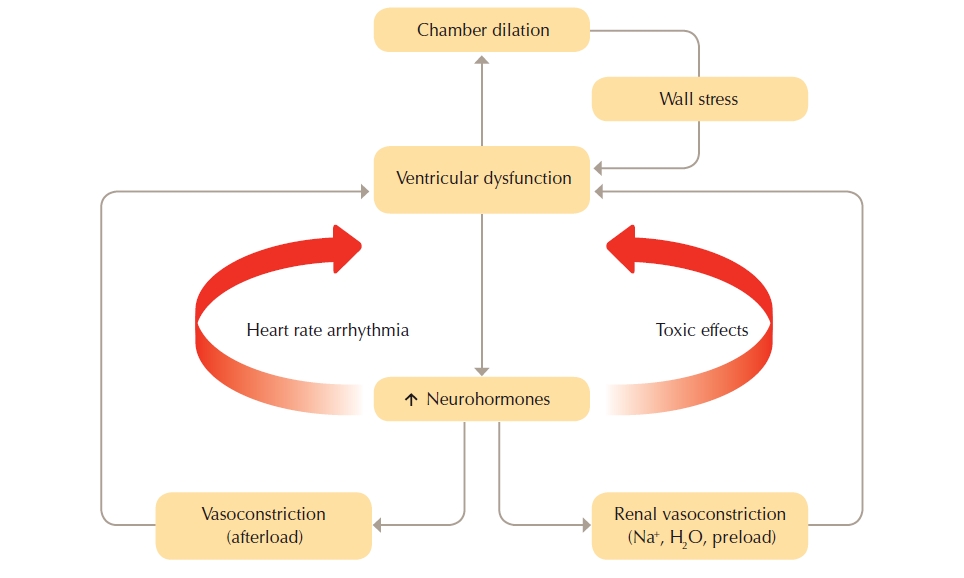
The cycle commences with insult to the myocardium. Neurohormonal compensatory activities are triggered when cardiac output declines.
Heart failure with Reduced Ejection Fraction (HFrEF)
Heart failure with reduced left ventricular systolic function resulting in poor cardiac output activates neurohormonal responses that over the long term can be either maladaptive or ineffectual. Many heart failure medications modify neurohormonal responses to break the vicious cycle of heart failure. See Combating the neurohormonal cycle in heart failure.
The neurohormonal systems involved in the cycle of heart failure include the sympathetic nervous system (SNS), renin-angiotensin-aldosterone system (RAAS) and the natriuretic peptide system (NPS).
The SNS and RAAS work to increase circulating blood volume through vasoconstriction and sodium and water retention. Over the long term SNS and RAAS over-activation may result in:
- Counterproductive neurohormonal adaptation as attempts to maintain arterial pressure advances deterioration in heart function.
- Raising levels of noradrenaline, angiotensin II and aldosterone, leading to chronic systemic and pulmonary vasoconstriction, cardiac hypertrophy and ischaemia, oedema, hyponatraemia and susceptibility to cardiac arrhythmia.
- Systemic alterations of renal, vascular and skeletal muscle function that further deteriorates cardiac performance. Renal impairment triggered by a decline in cardiac performance and major alteration in regional blood flow leading to change in skeletal muscle function as seen by general muscle weakness.
- Deteriorating blood flow and impaired muscle function that contribute to signs and symptoms such as salt and fluid retention, thirst and fatigue.
- Cardiac remodelling (changes in structure and shape) that further reduces cardiac output.
The NPS plays an important role in water and salt homeostasis by increasing sodium excretion in the urine. When the heart is under stress due to increased filling pressures, B-natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) are released. The NPS counter balances vasoconstriction, caused by the SNS and RAAS, by promoting vasodilatation and diuresis and can be effective in the early phases of heart failure. However, over the long term, NPS activation cannot effectively counter the adverse effects of over-activation of these systems.
Heart Failure with Preserved Ejection Fraction (HFpEF)
The pathophysiology of HFpEF is less clear than in HFrEF. In HFpEF, there is fluid overload and other symptoms without apparent reduction in left ventricular function. Current pathophysiological theories related to HFpEF include: coronary microvascular inflammation, central arterial stiffening, abnormalities in contractile and chronotropic reserve, and abnormalities in skeletal muscle oxygen delivery.
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators
 Pathophysiology
Pathophysiology
 Treatment & Management
Treatment & Management
 Exercise
Exercise
 Medications
Medications
 Psychosocial Issues
Psychosocial Issues
 Patient Education
Patient Education
 Behaviour Change
Behaviour Change
 Clinical Indicators
Clinical Indicators